|
Delighted to welcome Tommy who will be at Healthy Heights on Thursday - Tommy has been involved in Health and Nutrition Retail Management since 1985, and as a Qualified Nutritionist since 2000; starting his career in New York, United States and continuing his deep passion in the last decade in Sydney, Australia. So he understands first hand the importance of nutritious food, high quality supplements, and how they can make a monumental change to your life and completely overhaul your health and well-being.
Tommy has designed his own range of high quality sports and nutritional powders, with more than 30 years in the field of Nutrition, he is a well known and respected practitioner with peers and customers. His knowledge and love of health promotion with kindness and intention to improve the individuals journey, has earned him the respect of all who encounter him....... a great gain for our little apothecary... www.healthaddicts.com.au
0 Comments
 Winter is a time when many people are susceptible to colds, flu, and other respiratory illnesses. Fortunately, there are several herbs, foods and vitamins that can help boost the immune system and support overall health during the winter months. Here are a few suggestions that may be particularly helpful:
It is also important to talk to your healthcare provider before using any herbs or supplements, particularly if you are pregnant, nursing, or taking any medications
Lion's Mane mushrooms contain bioactive compounds such as beta-glucans, polysaccharides, and hericenones, which have been found to have a wide range of potential health benefits. These include boosting cognitive function and memory, reducing inflammation, improving digestive health, and supporting the immune system.
Studies have shown that Lion's Mane mushrooms may stimulate the growth of nerve cells and enhance the production of nerve growth factors, which can help protect and regenerate brain cells. This makes it a potentially promising natural remedy for neurological conditions such as Alzheimer's disease and Parkinson's disease. Lion's Mane mushrooms are also rich in antioxidants, which can help reduce oxidative stress and inflammation in the body. This can lead to improved cardiovascular health, reduced risk of cancer, and enhanced overall immunity. Overall, Lion's Mane mushrooms are a nutrient-dense superfood that may offer numerous potential health benefits. However, more research is needed to fully understand the mechanisms behind these benefits and to determine the optimal dosages and methods of consumption. There has been growing interest in the potential health benefits of Lion's Mane mushrooms, and several studies have been conducted to investigate these claims. Here are some examples of the research on Lion's Mane:
How to use Lions Mane: Lion's Mane mushroom can be used in a variety of ways and can be consumed on its own or combined with other supplements or foods. Here are some examples of how Lion's Mane can be used:
We recommend adding one scoop of powdered Lion's Mane Powder Mix into your favorite mug and mixing with hot water or a non-dairy milk, before adding in your morning tea or coffee to start your day with purpose and focus. Overall, Lion's Mane mushroom can be used in many different ways to provide potential health benefits. It is important to follow the recommended dosage and consult with a healthcare professional before taking any supplements. MAKE A SHAKE : Blueberries, beets and a brain boost, great for students. INGREDIENTS
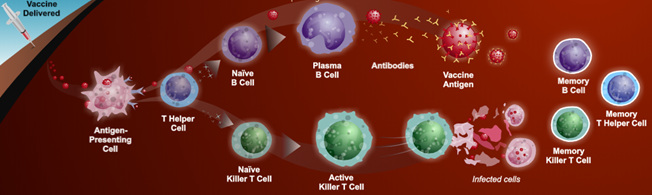
PREPARATION Mix all ingredients in a blender, blend until smooth! Compiled by Maxine White April 2023 for educational purposes only Pathophysiology In general we develop immunity to disease in two ways: Primary Infection: Pathogenic infectious agents stimulating a host immune system response (humoral and cell mediated immunity) that mitigates pathogenic infection including antibody production that promotes recovery and subsequent acquired immunity. Vaccination: Exposes the body to antigens that mimic disease causing pathogens, stimulating adaptive immunity and memory of the antigen without causing the disease. The process of acquiring vaccine immunity is referred to as immunisation.
Treatment RecommendationsCore RecommendationsHigh Bioavailability Zinc with Vitamin C Zinc and vitamin C to support the development, function and mediation of immune cells required to strengthen the immune response and enhance vaccine efficacy. Mechanism of Action/Clinical Research:
Vitamin D to modulate innate and adaptive immunity, necessary to illicit a strong immune response to acquire vaccine immunity and increase host resistance to infection. Mechanism of Action/Clinical Research:
For adults and children over 12 years: Mechanism of Action/Clinical Research:
References
[1] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [2] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [3] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [4] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [5] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [6] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [7] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [8] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021: cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [9] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [10] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [11] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [12] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [13] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [14] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [15] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [16] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [17] Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019 Mar 27;10:594. doi: 10.3389/fimmu.2019.00594. [18] Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. doi: 10.1038/nrd.2017.243. [19] Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. doi: 10.1038/nrd.2017.243. [20] Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018 Sep 19;9:1963. doi: 10.3389/fimmu.2018.01963. [21] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [22] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [23] Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, et al. Immunosenescence and challenges of vaccination against influenza in the aging Population. Aging Dis. 2012 Feb;3(1):68-90. PMID: 22500272. [24] Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016 Oct;46(10):2286-2301. doi: 10.1002/eji.201546178. [25] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [26] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [27] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [28] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [29] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [30] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [31] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [32] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [33] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [34] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [35] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [36] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [37] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [38] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [39] Rayman MP, Calder PC. Optimising COVID-19 vaccine efficacy by ensuring nutritional adequacy. Br J Nutr. 2021 Jan 28:1-2. doi: 10.1017/S0007114521000386. [40] Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020 Jan 16;12(1):236. doi: 10.3390/nu12010236. [41] Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020 May 20;3(1):74-92. doi: 10.1136/bmjnph-2020-000085. [42] Calder PC. Feeding the immune system. Proc Nutr Soc. 2013 Aug;72(3):299-309. doi: 10.1017/S0029665113001286. [43] Lee MD, Lin CH, Lei WT, Chang HY, Lee HC, Yeung CY, et al. Does Vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients. 2018 Mar 26;10(4):409. doi: 10.3390/nu10040409. [44] Ruggiero A, Battista A, Coccia P, Attinà G, Riccardi R. How to manage vaccinations in children with cancer. Pediatr Blood Cancer. 2011 Dec 15;57(7):1104-8. doi: 10.1002/pbc.23333. [45] Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020 Oct;45:102439. doi: 10.1016/j.msard.2020.102439. [46] Williamson EM, Chahin S, Berger JR. Vaccines in multiple sclerosis. Curr Neurol Neurosci Rep. 2016 Apr;16(4):36. doi: 10.1007/s11910-016-0637-6. [47] Australian Department of Government Health. Australian immunisation handbook: Vaccination for people who are immunocompromised [Internet]. Woden Town Centre, ACT: 2020 [updated 2020 Jul 3;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-for-special-risk-groups/vaccination-for-people-who-are-immunocompromised. [48] Williamson EM, Chahin S, Berger JR. Vaccines in multiple sclerosis. Curr Neurol Neurosci Rep. 2016 Apr;16(4):36. doi: 10.1007/s11910-016-0637-6. [49] Arunakumari PS, Kalburgi S, Sahare A, Vaccination in pregnancy. Obset Gynecol. 2015 Oct;17(4):257-63. doi: https://doi.org/10.1111/tog.12225. [50] Australian Department of Government Health. Australian immunisation handbook: Table. Vaccines that are routinely recommended in pregnancy: inactivated vaccines [Internet]. Woden Town Centre, ACT: 2018 [updated 2018 Jun 5;cited 2021 Mar 3]. Available from: https://immunisationhandbook.health.gov.au/resources/handbook-tables/table-vaccines-that-are-routinely-recommended-in-pregnancy-inactivated. [51] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [52] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [53] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [54] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [55] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [56] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [57] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [58] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [59] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [60] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [61] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [62] Braun L, Cohen M. Herbs and natural supplements: an evidence-based guide. 3rd ed. Sydney (AU): Elsevier/Churchill Livingstone; 2010. p. 1037-51. [63] Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021 Jan;143:1-9. doi: 10.1016/j.maturitas.2020.08.003. [64] Das R, Jobayer Chisti M, Ahshanul Haque M, Ashraful Alam M, Das S, Mahfuz M, et al. Evaluating association of vaccine response to low serum zinc and vitamin D levels in children of a birth cohort study in Dhaka. Vaccine. 2021 Jan 3;39(1):59-67. doi: 10.1016/j.vaccine.2020.10.048. [65] Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015 Mar;36(1):91-9.doi:10.1016/j.ccm.2014.11.002. [66] Maggini S, Beveridge S, Suter M. A combination of high-dose vitamin C plus zinc for the common cold. J Int Med Res. 2012;40(1):28-42. [67] Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011 Mar 28;17(12):1531-7. doi: 10.3748/wjg.v17.i12. [68] Abobaker A, Alzwi A, Alraied AHA. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol Rep. 2020 Dec;72(6):1517-1528. doi: 10.1007/s43440-020-00176-1. [69] Jayachandran M, Rani P, Arivazhagan P, Panneerselvam C. Neutrophil phagocytic function and humoral immune response with reference to ascorbate supplementation in aging humans. J Anti-Aging Med. 2000;3(1):37–42. doi: 10.1089/rej.1.2000.3.37. [70] Fisher SA, Rahimzadeh M, Brierley C, Gration B, Doree C, Kimber CE, et al. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS One. 2019 Sep 24;14(9):e0222313. doi: 10.1371/journal.pone.0222313. [71] Mocanu V, Oboroceanu T, Zugun-Eloae F. Current status in vitamin D and regulatory T cells--immunological implications. Rev Med Chir Soc Med Nat Iasi. 2013 Oct-Dec;117(4):965-73. PMID: 24502077. [72] Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56(7):2143-9. [73] Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56(7):2143-9. [74] Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011 Mar 28;17(12):1531-7. doi: 10.3748/wjg.v17.i12. [75] Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S-88S. [76] Zitt E, Sprenger-Mähr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine. 2012 Jan 20;30(5):931-5. doi: 10.1016/j.vaccine.2011.11.086. [77] Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017 Oct 27;9(11):1175. doi: 10.3390/nu9111175. [78] Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017 Oct 27;9(11):1175. doi: 10.3390/nu9111175. [79] Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017 Oct 27;9(11):1175. doi: 10.3390/nu9111175. [80] Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010 Feb 2;5(2):e9009. doi:10.1371/journal.pone.0009009. [81] Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010 Jun 1;50(6):597-602. doi: 10.1111/j.1472-765X.2010.02844.x. [82] Miyazawa K, Yoda K, Kawase M, Harata G, He F. Influence of orally administered Lactobacillus GG on respiratory immune response in a murine model of diet-induced obesity. Microbiol Immunol. 2015 Feb;59(2):99-103. doi: 10.1111/1348-0421.12226. [83] Ahrén IL, Hillman M, Nordström EA, Larsson N, Niskanen TM. Fewer community-acquired colds with daily consumption of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. A randomized, placebo-controlled clinical trial. J Nutr. 2021 Jan 4;151(1):214-222. doi: 10.1093/jn/nxaa353. [84] Berggren A, Lazou Ahrén I, Larsson N, Önning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011 Apr;50(3):203-10. doi: 10.1007/s00394-010-0127-6. [85] Rask C, Adlerberth I, Berggren A, Ahrén IL, Wold AE. Differential effect on cell-mediated immunity in human volunteers after intake of different lactobacilli. Clin Exp Immunol. 2013 May;172(2):321-32. doi: 10.1111/cei.12055. [86] Lee Y, Salminen S. Handbook of Probiotics and Prebiotics. 2nd ed. Hoboken: John Wiley & Sons; 2009. p.469-73. [87] Schultz M, Linde HJ, Lehn N, Zimmermann K, Grossmann J, Falk W, et al. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. J Dairy Res. 2003 May;70(2):165-73. [88] Wang H, Zhang L, Xu S, Pan J, Zhang Q, Lu R. Surface-layer protein from lactobacillus acidophilus NCFM inhibits lipopolysaccharide-induced inflammation through MAPK and NF-κB signaling pathways in RAW264.7 cells. J Agric Food Chem. 2018 Jul 25;66(29):7655-7662. doi: 10.1021/acs.jafc.8b02012. [89] Wei M, Wang Z, Liu H, Jiang H, Wang M, Liang S, et al. Probiotic Bifidobacterium animalis subsp. lactis Bi-07 alleviates bacterial translocation and ameliorates microinflammation in experimental uraemia. Nephrology. 2014 Aug;19(8):500-6. doi: 10.1111/nep.12272. [90] Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009 Aug;124(2):e172-9. doi: 10.1542/peds.2008-2666. [91] Fastinger ND, Karr-Lilienthal LK, Spears JK, Swanson KS, Zinn KE, Nava GM, et al. A novel resistant maltodextrin alters gastrointestinal tolerance factors, fecal characteristics, and fecal microbiota in healthy adult humans. J Am Coll Nutr. 2008 Apr;27(2):356-66. PMID: 18689571. [92] Lee Y, Salminen S. Handbook of Probiotics and Prebiotics. 2nd ed. Hoboken: John Wiley & Sons; 2009. p.469-73. [93] Pärtty A, Lehtonen L, Kalliomäki M, Salminen S, Isolauri E. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial. Pediatr Res. 2015 Oct;78(4):470-5. [94] Jungersen M, Wind A, Johansen E, Christensen JE, Stuer-Lauridsen B, Eskesen D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms. 2014 Mar;2(2):92-110. [95] Wu BB, Yang Y, Xu X, Wang WP. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr. 2016 May;12(2):177-82. [96] Inoue Y, Iwabuchi N, Xiao JZ, Yaeshima T, Iwatsuki K. Suppressive effects of bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol Pharm Bull. 2009 Apr;32(4):760-3. [97] Roman BE, Beli E, Duriancik DM, Gardner EM. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res. 2013 Jan;33(1):12-7. doi: 10.1016/j.nutres.2012.11.001. [98] Wang S, Welte T, Fang H, Chang GJ, Born WK, O'Brien RL, et al. Oral administration of active hexose correlated compound enhances host resistance to West Nile encephalitis in mice. J Nutr. 2009 Mar;139(3):598-602. doi: 10.3945/jn.108.100297. [99] Nogusa S, Gerbino J, Ritz BW. Low-dose supplementation with active hexose correlated compound improves the immune response to acute influenza infection in C57BL/6 mice. Nutr Res. 2009 Feb;29(2):139-43. doi: 10.1016/j.nutres.2009.01.005. [100] Ritz BW, Nogusa S, Ackerman EA, Gardner EM. Supplementation with active hexose correlated compound increases the innate immune response of young mice to primary influenza infection. J Nutr. 2006 Nov;136(11):2868-73. PMID: 17056815. [101] Terakawa N, Matsui Y, Satoi S, Yanagimoto H, Takahashi K, Yamamoto T, et al. Immunological effect of active hexose correlated compound (AHCC) in healthy volunteers: a double-blind, placebo-controlled trial. Nutr Cancer. 2008;60(5):643-51. doi:10.1080/01635580801993280. [102] Yin Z, Fujii H, Walshe T. Effects of active hexose correlated compound on frequency of CD4+ and CD8+ T cells producing interferon and/or tumour necrosis factor-alpha in healthy adults. Hum Immunol. 2010 Dec;71(12):1187-90. doi:10.1016/j.humimm.2010.08.006. [103] Roman BE, Beli E, Duriancik DM, Gardner EM. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res. 2013 Jan;33(1):12-7. doi: 10.1016/j.nutres.2012.11.001. [104] Smith JA, Mathew L, Gaikwad A, Rech B, Burney MN, Faro JP, et al. From bench to bedside: evaluation of AHCC supplementation to modulate the host immunity to clear high-risk human papillomavirus infections. Front Oncol. 2019 Mar 20;9:173. doi:10.3389/fonc.2019.00173. [105] Love KM, Barnett RE, Holbrook I, Sonnenfeld G, Fujii H, Sun B, et al. A natural immune modulator attenuates stress hormone and catecholamine concentrations in polymicrobial peritonitis. J Trauma Acute Care Surg. 2013 Jun;74(6):1411-8. doi: 10.1097/TA.0b013e31829215b1. [106] Love KM, Barnett RE, Holbrook I, Sonnenfeld G, Fujii H, Sun B, et al. A natural immune modulator attenuates stress hormone and catecholamine concentrations in polymicrobial peritonitis. J Trauma Acute Care Surg. 2013 Jun;74(6):1411-8. doi: 10.1097/TA.0b013e31829215b1. [107] Sahu P, Giri DD, Singh R, Pandey P, Gupta S, Shrivastava AK, et al. Therapeutic and medicinal uses of Aloe vera: a review. Pharmacol Pharm. 2013;4(8):599-610. doi: 10.4236/pp.2013.48086 . [108] Raja AF, Ali F, Khan IA, Shawl AS, Arora DS, Shah BA, et al. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011 Mar 16;11:54. doi: 10.1186/1471-2180-11-54. [109] Akisu M, Baka M, Huseyinov A, Kultursay N. The role of dietary supplementation with L-glutamine in inflammatory mediator release and intestinal injury in hypoxia/reoxygenation-induced experimental necrotizing enterocolitis. Ann Nutr Metab. 2003;47(6):262-6. PMID: 14520021. [110] Kelly GS. Larch arabinogalactan: clinical relevance of a novel immune-enhancing polysaccharide. Altern Med Rev. 1999;4(2):96-103. [111] Cario E, Jung S, Harder D'Heureuse J, Schulte C, Sturm A, Wiedenmann B, et al. Effects of exogenous zinc supplementation on intestinal epithelial repair in vitro. Eur J Clin Invest. 2000 May;30(5):419-28. PMID: 10809902. [112] Rayman MP, Calder PC. Optimising COVID-19 vaccine efficacy by ensuring nutritional adequacy. Br J Nutr. 2021 Jan 28:1-2. doi: 10.1017/S0007114521000386. [113] Gibson A, Edgar JD, Neville CE, Gilchrist SE, McKinley MC, Patterson CC, et al. Effect of fruit and vegetable consumption on immune function in older people: a randomized controlled trial. Am J Clin Nutr. 2012 Dec;96(6):1429-36. doi: 10.3945/ajcn.112.039057. [114] Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14(4):245-54. doi:10.2174/1871530314666140922153350. [115] Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14(4):245-54. doi:10.2174/1871530314666140922153350. [116] Haß U, Herpich C, Norman K. Anti-inflammatory diets and fatigue. Nutrients. 2019 Sep 30;11(10):2315. doi:10.3390/nu11102315. [117] Victorian State Government, Better Health Channel. Immunisation – side effects [Internet]. Melbourne VIC: Victorian State Government, Better Health Channel; 2018 [updated 2018 Apr 19; cited 2021 Mar 12]. Available from: https://www.betterhealth.vic.gov.au/health/healthyliving/immunisation-side-effects?viewAsPdf=true. [118] Victoria State Government, Better Health Channel. Fatigue [Internet]. Melbourne VIC: Victoria State Government, Better Health Channel; 2015 [updated 2015 Jun; cited 2020 Feb 12]. Available from: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/fatigue. [119] Victoria State Government, Better Health Channel. Fatigue [Internet]. Melbourne VIC: Victoria State Government, Better Health Channel; 2015 [updated 2015 Jun; cited 2020 Feb 12]. Available from: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/fatigue. [120] Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018 Apr 16;9:648. doi:10.3389/fimmu.2018.00648. [121] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [122] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [123] Ring J, Klimek L, Worm M. Adrenaline in the acute treatment of anaphylaxis. Dtsch Arztebl Int. 2018 Aug 6;115(31-32):528-534. doi: 10.3238/arztebl.2018.0528. This document discusses the mechanisms of action of a number of different botanical and nutraceutical agents. These agents can be considered as immunoadjuvants, defined as substances that act to accelerate, prolong, or enhance antigen-specific immune responses by potentiating or modulating the immune response.[1]
A coronavirus such as SARS-CoV-2 can be deadly because of its ability to stimulate a part of the innate immune response called the inflammasome, which can cause uncontrolled release of pro-inflammatory cytokines, leading to cytokine storm and severe, sometimes irreversible, damage to respiratory epithelium.[2] The SARS-CoV-2 virus has been shown to activate the NLRP3 inflammasome.[3,4] A 2016 review article[5] entitled “Natural compounds as regulators of NLRP3 inflammasome-mediated IL-beta production” notes that “resveratrol, curcumin, EGCG [epigallocatechin gallate], and quercetin are potent inhibitors of NLRP3 inflammasome-mediated IL-1beta production, typically acting at more than one element of the involved pathways. However, it should be noted that these polyphenols have an even much broader biological effect, as they influence a variety of pathways.” For example, these polyphenols modulate NF-kB upregulation, which is useful to counteract the COVID-19 ’hyper-inflammation.[6] A preprint released on March 23, 2020, identified the ability of plant bioactive compounds to inhibit the COVID-19 main protease (Mpro),[7] which is necessary for viral replication. There is much excitement surrounding the recent identification of Mpro, and it is a current potential pharmaceutical drug target. Kaempferol, quercetin, luteolin-7-glucoside, demethoxycurcumin, naringenin, apigenin-7glucoside, oleuropein, curcumin, catechin, and epicatechin-gallate were the natural compounds that appeared to have the best potential to act as COVID-19 Mpro inhibitors. Though further research is necessary to prove their efficacy, this study provides the biologic plausibility and mechanistic support (SARS-CoV-2 protease inhibition) to justify their use. For these reasons, we recommend the following compounds, at standard dosages, to prevent activation of the NLRP3 inflammasome, to decrease NF-kB activation, and to potentially inhibit SARS-CoV-2 replication. There is no literature to support a regimen of a single vs. multiple agents. Our recommendation is to use higher dosing and/or multiple agents when patient contextual factors (e.g., patient desire, pre-existing inflammation, multiple co-morbidities, higher risk, etc.) and/or therapeutic decision-making warrant such use. Recommended Interventions QUERCETIN Quercetin has been shown to have antiviral effects against both RNA (e.g., influenza and coronavirus) and DNA viruses (e.g., herpesvirus). Quercetin has a pleiotropic role as an antioxidant and anti-inflammatory, modulating signaling pathways that are associated with post-transcriptional modulators affecting post-viral healing.[8] Mechanism(s) of action against non-COVID-19 viruses Promote viral eradication or inactivation:[9],[10],[11],[12],[13] •Inhibition of viral replication Favorably modulate viral-induced pathological cellular processes: Modulation of NLRP3 inflammasome activation[5],[14],[15] Mechanistically promote resolution of collateral damage and restoration of function: Modulation of mast cell stabilization (anti-fibrotic) CURCUMIN Curcumin has been shown to modulate the NLRP3 inflammasome,5 and a preprint suggests that curcumin can target the SARS-CoV-2 main protease to reduce viral replication.18 Mechanism(s) of action against non-COVID-19 viruses Favorably modulate viral-induced pathological cellular processes: Modulation of NLRP3 inflammasome activation[5],[19],[20],[21] EPIGALLOCATECHIN GALLATE (EGCG) Green tea, in addition to modulating the NLRP3 inflammasome and, based on a preprint, potentially targeting the SARS-CoV-2 main protease (Mpro)7 to reduce viral replication, has also been shown to prevent influenza in healthcare workers.28 Mechanism(s) of action against non-COVID-19 viruses Favorably modulate viral-induced pathological cellular processes: Modulation of NLRP3 inflammasome activation[5],[28],[29] N-ACETYLCYSTEINE (NAC) N-acetylcysteine promotes glutathione production, which has been shown to be protective in rodents infected with influenza. In a little-noticed six-month controlled clinical study enrolling 262 primarily elderly subjects, those receiving 600 mg NAC twice daily, as opposed to those receiving placebo, experienced significantly fewer influenza-like episodes and days of bed confinement.[36] Mechanism(s) of action against non-COVID-19 viruses:[36]Favorably modulate cellular defense and repair mechanisms: Hypothetical: repletion of glutathione and cysteine Outcomes data supporting their mitigating effects on illness from other viral strains Reduce progression from colonization to illness Reduce the severity and duration of acute symptoms RESVERATROL Resveratrol, a naturally occurring polyphenol, shows many beneficial health effects. It has been shown to modulate the NLRP3 inflammasome.[5] In addition, resveratrol was shown to have in vitro activity against MERS-CoV.[43] Mechanism(s) of action against non-COVID-19 viruses Favorably modulate viral-induced pathological cellular processes Modulation of NLRP3 inflammasome activation[5] Outcomes data supporting their mitigating effects on illness from other viral strainsMERS-CoV[43] Influenza[44],[45] VITAMIN D Activated vitamin D,1,25(OH) D, a steroid hormone, is an immune system modulator that reduces the expression of inflammatory cytokines and increases macrophage function. Vitamin D also stimulates the expression of potent antimicrobial peptides (AMPs), which exist in neutrophils, monocytes, natural killer cells, and epithelial cells of the respiratory tract.[54] Vitamin D increases anti-pathogen peptides through defensins and has a dual effect due to suppressing superinfection. Evidence suggests vitamin D supplementation may prevent upper respiratory infections.[55] However, there is some controversy as to whether it should be used and the laboratory value that should be achieved. Research suggests that concerns about vitamin D (increased IL-1beta in cell culture) are not seen clinically. The guidance we suggest is that a laboratory range of >50 and < 80ng/mL serum 25-hydroxy vitamin D may help to mitigate morbidity from COVID-19 infection. Mechanism(s) of action against non-COVID-19 viruses[55],[56],[57],[58],[59],[60],[61],[62],[63],[64],[65],[66],[67],[68],[69],[70],[71],[72],[73],[74],[75],[76],[77],[78] Favorably modulate cellular defense and repair mechanisms: Activation of macrophages Stimulation of anti-microbial peptides Modulation of defensins Modulation of TH17 cells Favorably modulate viral-induced pathological cellular processes: Reduction in cytokine expression Modulation of TGF beta Outcomes data supporting their mitigating effects on illness from other viral strains Reduce progression from colonization to illness Reduce the severity and duration of acute symptoms and complications VITAMIN A Vitamin A is a micronutrient that is crucial for maintaining vision, promoting growth and development, and protecting epithelium and mucus integrity in the body. Vitamin A is known as an anti-inflammation vitamin because of its critical role in enhancing immune function. Vitamin A is involved in the development of the immune system and plays regulatory roles in cellular immune responses and humoral immune processes through the modulation of T helper cells, sIgA, and cytokine production. Vitamin A has demonstrated a therapeutic effect in the treatment of various infectious diseases.[95] Mechanism(s) of action against non-COVID-19 viruses [95],[96]Favorably modulate cellular defense and repair mechanisms: Modulation of T helper cells Modulation of sIgA Favorably modulate viral-induced pathological cellular processes: Modulation of cytokine production Outcomes data supporting their mitigating effects on illness from other viral strainsNo data available ELDERBERRY Elderberry (Sambucus nigra) is seen in many medicinal preparations and has widespread historical use as an anti-viral herb.[103] Based on animal research, elderberry is likely most effective in the prevention of and early infection with respiratory viruses.[104] One in-vitro study reported an increase in TNF-alpha levels related to a specific commercial preparation of elderberry[105] leading some to caution that its use could initiate a “cytokine storm.” However, these data were not confirmed when the same group performed similar studies, which were published in 2002.[106] Therefore, these data suggest it is highly implausible that consumption of properly prepared elderberry products (from berries or flowers) would contribute to an adverse outcome related to overproduction of cytokines or lead to an adverse response in someone infected with COVID-19. Mechanism(s) of action against non-COVID-19 viruses[103],[107],[108],[109],[110],[111],[112] Favorably modulate cellular defense and repair mechanisms Favorably modulate viral-induced pathological cellular processes Outcomes data supporting their mitigating effects on illness from other viral strainsNo data available PALMITOYLETHANOLAMIDE (PEA) PEA is a naturally occurring anti-inflammatory palmitic acid derivative that interfaces with the endocannabinoid system. There was a significantly favorable outcome in five of six double blind placebo-controlled trials looking at acute respiratory disease due to influenza.[115] Dosing was generally 600 mg three times daily for up to three weeks. There are multiple mechanisms of action associated with PEA, from inhibition of TNF-alpha and NF-kB to mast cell stabilization. In influenza, it is thought that PEA works by attenuating the potentially fatal cytokine storm. Mechanism(s) of action against non-COVID-19 viruses[115] Favorably modulate cellular defense and repair mechanisms Favorably modulate viral-induced pathological cellular processes VITAMIN C Vitamin C contributes to immune defense by supporting various cellular functions of both the innate and adaptive immune system. Vitamin C accumulates in phagocytic cells, such as neutrophils, and can enhance chemotaxis, phagocytosis, generation of reactive oxygen species, and ultimately microbial killing. Supplementation with vitamin C appears to be able to both prevent and treat respiratory and systemic infections.[120] Vitamin C has been used in hospital ICUs to treat COVID-19 infection. Mechanism(s) of action against non-COVID-19 viruses [120] Favorably modulate cellular defense and repair mechanisms Favorably modulate viral-induced pathological cellular processes ZINC Zinc contributes to immune defense by supporting various cellular functions of both the innate and adaptive immune system. There is also evidence that it suppresses viral attachment and replication. Zinc deficiency is common, especially in those populations most at risk for severe COVID-19 infections, and it is challenging to accurately diagnosis with laboratory measures. Supplementation with zinc is supported by evidence that it both prevents viral infections and reduces their severity and duration. Moreover, it has been shown to reduce the risk of lower respiratory infection, which may be of particular significance in the context of COVID-19. Mechanism(s) of action against non-COVID-19 viruses120,121,122,123,124,125,126,127 Favorably modulate innate and adaptive immune system Favorably modulate viral-induced pathological cellular processes, attachment, and replication www.ifm.org/news-insights/the-functional-medicine-approach-to-covid-19-virus-specific-nutraceutical-and-botanical-agents/ This resource is only intended to identify nutraceutical and botanical agents that may boost your immune system. It is not meant to recommend any treatments, nor have any of these been proven effective against COVID-19. None of these practices are intended to be used in lieu of other recommended treatments. Always consult your physician or healthcare provider prior to initiation. For up-to-date information on COVID-19, please consult the Centers for Disease Control and Prevention at www.cdc.gov. Substantial number of older adults at risk of vitamin B12 and folate deficiency, study shows5/1/2020 A study by researchers from The Irish Longitudinal Study on Ageing (TILDA) at Trinity College Dublin, Ireland, has shown for the first time that a substantial number of adults over 50 are at risk of deficiency in vitamin B12 and folate (the natural vitamin linked to the dietary supplement, folic acid).
The researchers found that one in eight adults in Ireland are deficient in vitamin B12; one in seven are deficient in folate; and there are variations in deficiency across different provinces in Ireland, in addition to variations dependent on health, lifestyle and the time of year measured. The findings form part of the largest representative study of its kind conducted among older persons in Ireland and have just been published in the prestigious journal, British Journal of Nutrition. Both vitamin B12 and folate are essential for nerve function, brain health and the production of red blood cells and DNA. Numerous studies have shown that low nutritional status of folate and B12 are linked to poor long-term health, especially among older people. In Ireland, fortification of food products is voluntary and some foods (such as ready-to-eat cereals) are enriched with micronutrients such as folic acid, though this is inconsistent between products fortified and over time, resulting in haphazard exposure. There have been repeated calls for an official policy of mandatory fortification of staple foods such as bread, with folic acid, to reduce the occurrence of neural tube defects in babies. Such a policy would also reduce the prevalence of folate deficiency in older adults who are most at risk. Before this can occur, however, comprehensive information is needed on the prevalence and determinates of deficiency. Our study suggests that the current custom of voluntary food fortification is ineffective in preventing deficiency or low status of these vitamins among older people. The results are of relevance not just for Ireland but for all countries that do not have mandatory fortification. Key findings:
Commenting on the significance of the research, lead author of the study and Research Fellow at TILDA, Dr Eamon Laird, said: "This is the largest representative and most comprehensive study of vitamin B12 and folate status in older adults ever conducted in Ireland. There are striking differences in the prevalence of deficiency across different lifestyle factors such as obesity and smoking - both of which are modifiable risk factors. Our findings will provide useful data to help inform public health policy -particularly regarding the proposition of mandatory folic acid and/or vitamin B12 fortification. To place our findings in context, in a country such as the United States where mandatory folic acid fortification occurs, rates of low folate status are around 1.2% in older adults compared with 15% in Ireland." Professor Anne Molloy, senior author of the study noted: "This study shows a surprising level of inadequate folate among older persons, despite many years of voluntary folic acid fortification of certain foods on the Irish market. Concerns relating to excessive folic acid intake, particularly in older people, have been at the heart of current debates regarding the risks of population-wide folic acid fortification. However, in countries such as the US, mandatory folic acid food fortification for the past 20 years has prevented millions of cases of folate deficiency without any proven adverse effects. Irish public health authorities need to act on the facts from studies such as ours." Professor Rose Anne Kenny, Principal Investigator of TILDA, said: "The high rates of B-vitamin deficiency seen in the older adult population are of concern and, given that this can be easily treated with fortification, this has significant policy and practice implications for Government and health services. TILDA has consistently assisted policy makers by providing strong evidence based data on which to make recommendations but also by assisting with information of most vulnerable people and therefore those who should be targeted." Source: Trinity College Dublin β-Glucans have been shown to activate pattern recognition receptors expressed on immune cells, such as macrophages, dendritic cells, neutrophils and lymphocytes [1,3]. Additionally, the enhancement of natural killer (NK) cell activity by β-glucan has been reported to play an important role in immune potency and to have anti-carcinogenic effects in in vitro and in vivo studies [4,5]. The immune stimulation and antitumoral activities of these β-glucans have been thought to be caused only by the β-1,3-glucans [6]. However, several types of β-1,3-glucans from different sources have been shown to result in a variety of different immune responses.
https://www-ncbi-nlm-nih-gov.ezproxy.une.edu.au/pubmed/28194264 The Top 10 Blood Tests for Vegans
by Doctor J.E Williams By Dr. J.E. Williams I started my own personal experiment with vegetarianism and vegan lifestyle in 1972, and I also conceived and raised children as vegetarians (until they were pre-teen). I have 30 years of clinical experience in natural medicine, and for 25 years, I was a busy clinician in Southern California. Thus, I have earned my credentials and have seen it all. I know through all of this that if you want to get your cholesterol and LDL (“bad” cholesterol) down to bare bones levels, go vegan. If you want to boost your folic acid and antioxidant levels to new heights, eat more plants. It is the same with reducing your risk for a heart attack to zero, and preventing many types of cancer. But in some aspects, depending on their diet, vegetarians and vegans are vulnerable. Today I want to discuss the basic laboratory tests most important for plant-based diets. Let’s look at the 10 most helpful ones for evaluating deficiencies and the consequences of not having adequate levels of certain nutrients. 1. CBC - Complete Blood Count with Differential and Platelets: This group of tests tells if you are anemic, immune deficient, or have an infection or allergies. Low RBC (red blood count), hemoglobin, and hematocrit are signs of anemia. The CBC helps determine your general health status. If have fatigue or weakness, or suspect an infection, this test can help determine what is the cause. 2. CMP - Comprehensive Metabolic Panel: The CMP is a group of 14 tests that provides information about the status of your kidneys, liver, and electrolyte and acid/base balance, as well as of your blood sugar (glucose) and blood proteins (total protein, albumin, and globulin). Abnormal results, especially combinations of abnormal results, indicate a problem that needs to be addressed. Total protein below 6.5 and albumin below 3.9 are signs of protein deficiency. Glucose (blood sugar) is also tested in this panel. It is uncommon for plant-based eaters to be diabetic. Some times, however, glucose can be too low, suggesting hypoglycemia. 3. Ferritin: This test helps assess iron stores in the body. It is useful in combination with an iron and TIBC to evaluate the severity of iron deficiency or overload. 4. Folic Acid: This test gives an idea of your level of folate. It is rarely low in plant-based diets. However, higher than normal levels, common in vegetarians and vegans, combined with low vitamin B12 levels, magnifies vitamin B deficiency in the body. The amount of folate inside the red blood cell (folate, RBC) may also be measured and is normally higher inside the cell than in the serum. 5. Homocysteine: An elevated homocysteine level helps determine B12 or folate deficiency. Elevated levels of homocysteine (above 10 micromoles/liter) are associated with atherosclerosis (hardening and narrowing of the arteries) and suggest an increased risk of heart attacks, strokes, blood clot formation, and Alzheimer’s disease. I want my patients to be lower than 9 micromoles/liter and optimally less than 6 micromoles/liter. 6. Iron: total and TIBC (total iron binding capacity): Vegetarians can have adequate iron levels if they eat quantities of iron-containing vegetables and fruits, like spinach and raisins. However, raw vegans often show low levels of red blood cells and iron deficiency in their tests. Early iron deficiency causes no physical effects, so you may not know you levels are going down; but, as hemoglobin levels drop below 10 g per deciliter, things can get challenging. As the iron-deficiency progresses, symptoms begin to develop, including fatigue and tiredness, weakness, dizziness, and headaches. As iron reserves continue to be depleted, you can experience shortness of breath, ringing in the ears (tinnitus), drowsiness, and irritability. 7. Lipid Profile: This group of tests measures your blood fats (total cholesterol, LDL, HDL, and triglycerides) to determine risk for coronary heart disease. Vegetarians typical have normal lipid profiles, but vegans may have cholesterol levels that are too low (less than 135 mg/dL). Cholesterol is essential for life. A waxy substance manufactured from raw materials supplied in the diet, it is used to produce hormones and cell membranes and is transported in the blood. Cholesterol is the primary building block for steroid hormones like estrogen and testosterone, and adequate levels are required for health. 8. MMA - Methylmalonic Acid, serum: MMA, along with homocysteine, help diagnose an early or mild B12 deficiency. If MMA and homocysteine levels are increased, then vitamin B12 deficiency may be present, indicating less available B12 at the tissue level. If only homocysteine is elevated, then folic acid may be low or not being metabolism properly. If MMA and homocysteine levels are normal, it is unlikely that there is a B12 deficiency. 9. Vitamin B12: Both B12 and folate are necessary for normal red blood cell formation, tissue and cellular repair, DNA synthesis, and for nerve health. A deficiency in either B12 or folate causes macrocytic anemia. Also called megaloblastic anemia, this type of anemia is characterized by the production of fewer – but larger – red blood cells called macrocytes, leading to fatigue, weakness, and all the other symptoms of anemia. If your levels are below 400 pg/mL, suspect B12 deficiency. I like my patients to be at least 600-900 pg/mL. 10. Vitamin D, 25-Hydroxy: This test determines vitamin D3 status. It tells if you are susceptible to bone weakness, bone malformation, or abnormal metabolism of calcium. Since vitamin D is a fat-soluble vitamin and absorbed from the intestine like dietary fat, low-fat diets are prone to vitamin D deficiency. Also, people with conditions that interfere with fat absorption, such as cystic fibrosis and Crohn’s disease, irritable bowel syndrome, and Celiac disease are not able to absorb enough Vitamin D. Dr. Williams’ Suggested Panels for Vegetarians/Vegans Complete Blood Count with Differential and Platelets Comprehensive Chemistry/Metabolic Panel Ferritin Folic Acid Homocysteine Iron, total and IBC Lipid Panel Methylmalonic Acid, Serum Vitamin B12 Vitamin D3, 25 Hydroxy A note from Bobby-Jo:
Recently I've been focusing on working with children with ADD, behavioural disorders, ADHD, anxiety, and sensory and sleep disturbances. On a personal level this area of naturopathic care is particularly close to my heart. Did you know it only takes 26 seconds for chemicals to enter the blood stream and start playing havoc within our bodies!? I have seen first hand the immediate behavioural changes within my own kids after consumption of food additives. I know all the letters, numbers and long unpronounceable names on food labels can seem confusing and complicated, however some are worse than others and I can help guide you to make informed choices. Making a few simple changes can make a significant difference to behaviour and general health. If this rings alarm bells for you or a little one you know, come in for a chat or book a consultation today. Rest assured there is a lot we can do with these issues from both a dietary and supplemental perspective. I'd love to work together towards better health for your family. Bobby-Jo Some common additives to avoid include: 102 Tartrazine, yellow #5, CI 19140 212 Potassium benzoate AVOID Some common additives to should be treated with caution including: 216 Propylparaben or Propyl-p-hydroxy-benzoate 218 Methylparaben or Methyl-p-hydroxy-benzoate And when it comes to 951 Aspartame.... well you’ll just have to come in and see me to find out more ; ) |
AuthorSNotes from the team: Maxine, Monique and Bobby-Jo Archives
May 2023
|


 RSS Feed
RSS Feed
