|
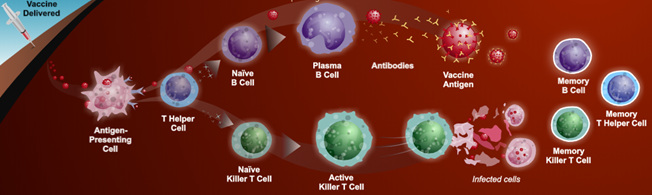
Pathophysiology In general we develop immunity to disease in two ways: Primary Infection: Pathogenic infectious agents stimulating a host immune system response (humoral and cell mediated immunity) that mitigates pathogenic infection including antibody production that promotes recovery and subsequent acquired immunity. Vaccination: Exposes the body to antigens that mimic disease causing pathogens, stimulating adaptive immunity and memory of the antigen without causing the disease. The process of acquiring vaccine immunity is referred to as immunisation.
Treatment RecommendationsCore RecommendationsHigh Bioavailability Zinc with Vitamin C Zinc and vitamin C to support the development, function and mediation of immune cells required to strengthen the immune response and enhance vaccine efficacy. Mechanism of Action/Clinical Research:
Vitamin D to modulate innate and adaptive immunity, necessary to illicit a strong immune response to acquire vaccine immunity and increase host resistance to infection. Mechanism of Action/Clinical Research:
For adults and children over 12 years: Mechanism of Action/Clinical Research:
References
[1] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [2] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [3] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [4] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [5] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [6] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [7] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [8] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021: cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [9] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [10] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [11] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [12] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [13] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [14] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [15] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [16] Centre for Disease Control and Prevention. Understanding how vaccines work [Internet]. Atlanta, Georgia: 2018 [updated 2018 Jul;cited 2021 Feb 18]. Available from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. [17] Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019 Mar 27;10:594. doi: 10.3389/fimmu.2019.00594. [18] Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. doi: 10.1038/nrd.2017.243. [19] Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. doi: 10.1038/nrd.2017.243. [20] Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018 Sep 19;9:1963. doi: 10.3389/fimmu.2018.01963. [21] The College of Physicians of Philadelphia. The history of vaccines [Internet]. Philadelphia USA: The College of Physicians of Philadelphia; 2021 [updated 2021;cited 2021 Feb 18]. Available from: https://www.historyofvaccines.org/content/how-vaccines-work. [22] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [23] Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, et al. Immunosenescence and challenges of vaccination against influenza in the aging Population. Aging Dis. 2012 Feb;3(1):68-90. PMID: 22500272. [24] Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016 Oct;46(10):2286-2301. doi: 10.1002/eji.201546178. [25] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [26] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [27] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [28] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [29] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [30] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [31] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [32] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [33] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [34] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [35] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [36] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [37] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [38] Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 13;32(2):e00084-18. doi: 10.1128/CMR.00084-18. [39] Rayman MP, Calder PC. Optimising COVID-19 vaccine efficacy by ensuring nutritional adequacy. Br J Nutr. 2021 Jan 28:1-2. doi: 10.1017/S0007114521000386. [40] Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020 Jan 16;12(1):236. doi: 10.3390/nu12010236. [41] Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020 May 20;3(1):74-92. doi: 10.1136/bmjnph-2020-000085. [42] Calder PC. Feeding the immune system. Proc Nutr Soc. 2013 Aug;72(3):299-309. doi: 10.1017/S0029665113001286. [43] Lee MD, Lin CH, Lei WT, Chang HY, Lee HC, Yeung CY, et al. Does Vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients. 2018 Mar 26;10(4):409. doi: 10.3390/nu10040409. [44] Ruggiero A, Battista A, Coccia P, Attinà G, Riccardi R. How to manage vaccinations in children with cancer. Pediatr Blood Cancer. 2011 Dec 15;57(7):1104-8. doi: 10.1002/pbc.23333. [45] Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020 Oct;45:102439. doi: 10.1016/j.msard.2020.102439. [46] Williamson EM, Chahin S, Berger JR. Vaccines in multiple sclerosis. Curr Neurol Neurosci Rep. 2016 Apr;16(4):36. doi: 10.1007/s11910-016-0637-6. [47] Australian Department of Government Health. Australian immunisation handbook: Vaccination for people who are immunocompromised [Internet]. Woden Town Centre, ACT: 2020 [updated 2020 Jul 3;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-for-special-risk-groups/vaccination-for-people-who-are-immunocompromised. [48] Williamson EM, Chahin S, Berger JR. Vaccines in multiple sclerosis. Curr Neurol Neurosci Rep. 2016 Apr;16(4):36. doi: 10.1007/s11910-016-0637-6. [49] Arunakumari PS, Kalburgi S, Sahare A, Vaccination in pregnancy. Obset Gynecol. 2015 Oct;17(4):257-63. doi: https://doi.org/10.1111/tog.12225. [50] Australian Department of Government Health. Australian immunisation handbook: Table. Vaccines that are routinely recommended in pregnancy: inactivated vaccines [Internet]. Woden Town Centre, ACT: 2018 [updated 2018 Jun 5;cited 2021 Mar 3]. Available from: https://immunisationhandbook.health.gov.au/resources/handbook-tables/table-vaccines-that-are-routinely-recommended-in-pregnancy-inactivated. [51] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [52] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [53] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [54] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [55] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [56] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [57] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [58] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [59] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [60] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [61] Australian Department of Government Health. Australian immunisation handbook: After vaccination [Internet]. Woden Town Centre, ACT: 2021 [updated 2021 Jan 13;cited 2021 Feb 18]. Available from: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination. [62] Braun L, Cohen M. Herbs and natural supplements: an evidence-based guide. 3rd ed. Sydney (AU): Elsevier/Churchill Livingstone; 2010. p. 1037-51. [63] Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021 Jan;143:1-9. doi: 10.1016/j.maturitas.2020.08.003. [64] Das R, Jobayer Chisti M, Ahshanul Haque M, Ashraful Alam M, Das S, Mahfuz M, et al. Evaluating association of vaccine response to low serum zinc and vitamin D levels in children of a birth cohort study in Dhaka. Vaccine. 2021 Jan 3;39(1):59-67. doi: 10.1016/j.vaccine.2020.10.048. [65] Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015 Mar;36(1):91-9.doi:10.1016/j.ccm.2014.11.002. [66] Maggini S, Beveridge S, Suter M. A combination of high-dose vitamin C plus zinc for the common cold. J Int Med Res. 2012;40(1):28-42. [67] Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011 Mar 28;17(12):1531-7. doi: 10.3748/wjg.v17.i12. [68] Abobaker A, Alzwi A, Alraied AHA. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol Rep. 2020 Dec;72(6):1517-1528. doi: 10.1007/s43440-020-00176-1. [69] Jayachandran M, Rani P, Arivazhagan P, Panneerselvam C. Neutrophil phagocytic function and humoral immune response with reference to ascorbate supplementation in aging humans. J Anti-Aging Med. 2000;3(1):37–42. doi: 10.1089/rej.1.2000.3.37. [70] Fisher SA, Rahimzadeh M, Brierley C, Gration B, Doree C, Kimber CE, et al. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS One. 2019 Sep 24;14(9):e0222313. doi: 10.1371/journal.pone.0222313. [71] Mocanu V, Oboroceanu T, Zugun-Eloae F. Current status in vitamin D and regulatory T cells--immunological implications. Rev Med Chir Soc Med Nat Iasi. 2013 Oct-Dec;117(4):965-73. PMID: 24502077. [72] Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56(7):2143-9. [73] Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56(7):2143-9. [74] Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011 Mar 28;17(12):1531-7. doi: 10.3748/wjg.v17.i12. [75] Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S-88S. [76] Zitt E, Sprenger-Mähr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine. 2012 Jan 20;30(5):931-5. doi: 10.1016/j.vaccine.2011.11.086. [77] Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017 Oct 27;9(11):1175. doi: 10.3390/nu9111175. [78] Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017 Oct 27;9(11):1175. doi: 10.3390/nu9111175. [79] Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017 Oct 27;9(11):1175. doi: 10.3390/nu9111175. [80] Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010 Feb 2;5(2):e9009. doi:10.1371/journal.pone.0009009. [81] Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010 Jun 1;50(6):597-602. doi: 10.1111/j.1472-765X.2010.02844.x. [82] Miyazawa K, Yoda K, Kawase M, Harata G, He F. Influence of orally administered Lactobacillus GG on respiratory immune response in a murine model of diet-induced obesity. Microbiol Immunol. 2015 Feb;59(2):99-103. doi: 10.1111/1348-0421.12226. [83] Ahrén IL, Hillman M, Nordström EA, Larsson N, Niskanen TM. Fewer community-acquired colds with daily consumption of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. A randomized, placebo-controlled clinical trial. J Nutr. 2021 Jan 4;151(1):214-222. doi: 10.1093/jn/nxaa353. [84] Berggren A, Lazou Ahrén I, Larsson N, Önning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011 Apr;50(3):203-10. doi: 10.1007/s00394-010-0127-6. [85] Rask C, Adlerberth I, Berggren A, Ahrén IL, Wold AE. Differential effect on cell-mediated immunity in human volunteers after intake of different lactobacilli. Clin Exp Immunol. 2013 May;172(2):321-32. doi: 10.1111/cei.12055. [86] Lee Y, Salminen S. Handbook of Probiotics and Prebiotics. 2nd ed. Hoboken: John Wiley & Sons; 2009. p.469-73. [87] Schultz M, Linde HJ, Lehn N, Zimmermann K, Grossmann J, Falk W, et al. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. J Dairy Res. 2003 May;70(2):165-73. [88] Wang H, Zhang L, Xu S, Pan J, Zhang Q, Lu R. Surface-layer protein from lactobacillus acidophilus NCFM inhibits lipopolysaccharide-induced inflammation through MAPK and NF-κB signaling pathways in RAW264.7 cells. J Agric Food Chem. 2018 Jul 25;66(29):7655-7662. doi: 10.1021/acs.jafc.8b02012. [89] Wei M, Wang Z, Liu H, Jiang H, Wang M, Liang S, et al. Probiotic Bifidobacterium animalis subsp. lactis Bi-07 alleviates bacterial translocation and ameliorates microinflammation in experimental uraemia. Nephrology. 2014 Aug;19(8):500-6. doi: 10.1111/nep.12272. [90] Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009 Aug;124(2):e172-9. doi: 10.1542/peds.2008-2666. [91] Fastinger ND, Karr-Lilienthal LK, Spears JK, Swanson KS, Zinn KE, Nava GM, et al. A novel resistant maltodextrin alters gastrointestinal tolerance factors, fecal characteristics, and fecal microbiota in healthy adult humans. J Am Coll Nutr. 2008 Apr;27(2):356-66. PMID: 18689571. [92] Lee Y, Salminen S. Handbook of Probiotics and Prebiotics. 2nd ed. Hoboken: John Wiley & Sons; 2009. p.469-73. [93] Pärtty A, Lehtonen L, Kalliomäki M, Salminen S, Isolauri E. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial. Pediatr Res. 2015 Oct;78(4):470-5. [94] Jungersen M, Wind A, Johansen E, Christensen JE, Stuer-Lauridsen B, Eskesen D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms. 2014 Mar;2(2):92-110. [95] Wu BB, Yang Y, Xu X, Wang WP. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr. 2016 May;12(2):177-82. [96] Inoue Y, Iwabuchi N, Xiao JZ, Yaeshima T, Iwatsuki K. Suppressive effects of bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol Pharm Bull. 2009 Apr;32(4):760-3. [97] Roman BE, Beli E, Duriancik DM, Gardner EM. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res. 2013 Jan;33(1):12-7. doi: 10.1016/j.nutres.2012.11.001. [98] Wang S, Welte T, Fang H, Chang GJ, Born WK, O'Brien RL, et al. Oral administration of active hexose correlated compound enhances host resistance to West Nile encephalitis in mice. J Nutr. 2009 Mar;139(3):598-602. doi: 10.3945/jn.108.100297. [99] Nogusa S, Gerbino J, Ritz BW. Low-dose supplementation with active hexose correlated compound improves the immune response to acute influenza infection in C57BL/6 mice. Nutr Res. 2009 Feb;29(2):139-43. doi: 10.1016/j.nutres.2009.01.005. [100] Ritz BW, Nogusa S, Ackerman EA, Gardner EM. Supplementation with active hexose correlated compound increases the innate immune response of young mice to primary influenza infection. J Nutr. 2006 Nov;136(11):2868-73. PMID: 17056815. [101] Terakawa N, Matsui Y, Satoi S, Yanagimoto H, Takahashi K, Yamamoto T, et al. Immunological effect of active hexose correlated compound (AHCC) in healthy volunteers: a double-blind, placebo-controlled trial. Nutr Cancer. 2008;60(5):643-51. doi:10.1080/01635580801993280. [102] Yin Z, Fujii H, Walshe T. Effects of active hexose correlated compound on frequency of CD4+ and CD8+ T cells producing interferon and/or tumour necrosis factor-alpha in healthy adults. Hum Immunol. 2010 Dec;71(12):1187-90. doi:10.1016/j.humimm.2010.08.006. [103] Roman BE, Beli E, Duriancik DM, Gardner EM. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res. 2013 Jan;33(1):12-7. doi: 10.1016/j.nutres.2012.11.001. [104] Smith JA, Mathew L, Gaikwad A, Rech B, Burney MN, Faro JP, et al. From bench to bedside: evaluation of AHCC supplementation to modulate the host immunity to clear high-risk human papillomavirus infections. Front Oncol. 2019 Mar 20;9:173. doi:10.3389/fonc.2019.00173. [105] Love KM, Barnett RE, Holbrook I, Sonnenfeld G, Fujii H, Sun B, et al. A natural immune modulator attenuates stress hormone and catecholamine concentrations in polymicrobial peritonitis. J Trauma Acute Care Surg. 2013 Jun;74(6):1411-8. doi: 10.1097/TA.0b013e31829215b1. [106] Love KM, Barnett RE, Holbrook I, Sonnenfeld G, Fujii H, Sun B, et al. A natural immune modulator attenuates stress hormone and catecholamine concentrations in polymicrobial peritonitis. J Trauma Acute Care Surg. 2013 Jun;74(6):1411-8. doi: 10.1097/TA.0b013e31829215b1. [107] Sahu P, Giri DD, Singh R, Pandey P, Gupta S, Shrivastava AK, et al. Therapeutic and medicinal uses of Aloe vera: a review. Pharmacol Pharm. 2013;4(8):599-610. doi: 10.4236/pp.2013.48086 . [108] Raja AF, Ali F, Khan IA, Shawl AS, Arora DS, Shah BA, et al. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011 Mar 16;11:54. doi: 10.1186/1471-2180-11-54. [109] Akisu M, Baka M, Huseyinov A, Kultursay N. The role of dietary supplementation with L-glutamine in inflammatory mediator release and intestinal injury in hypoxia/reoxygenation-induced experimental necrotizing enterocolitis. Ann Nutr Metab. 2003;47(6):262-6. PMID: 14520021. [110] Kelly GS. Larch arabinogalactan: clinical relevance of a novel immune-enhancing polysaccharide. Altern Med Rev. 1999;4(2):96-103. [111] Cario E, Jung S, Harder D'Heureuse J, Schulte C, Sturm A, Wiedenmann B, et al. Effects of exogenous zinc supplementation on intestinal epithelial repair in vitro. Eur J Clin Invest. 2000 May;30(5):419-28. PMID: 10809902. [112] Rayman MP, Calder PC. Optimising COVID-19 vaccine efficacy by ensuring nutritional adequacy. Br J Nutr. 2021 Jan 28:1-2. doi: 10.1017/S0007114521000386. [113] Gibson A, Edgar JD, Neville CE, Gilchrist SE, McKinley MC, Patterson CC, et al. Effect of fruit and vegetable consumption on immune function in older people: a randomized controlled trial. Am J Clin Nutr. 2012 Dec;96(6):1429-36. doi: 10.3945/ajcn.112.039057. [114] Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14(4):245-54. doi:10.2174/1871530314666140922153350. [115] Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14(4):245-54. doi:10.2174/1871530314666140922153350. [116] Haß U, Herpich C, Norman K. Anti-inflammatory diets and fatigue. Nutrients. 2019 Sep 30;11(10):2315. doi:10.3390/nu11102315. [117] Victorian State Government, Better Health Channel. Immunisation – side effects [Internet]. Melbourne VIC: Victorian State Government, Better Health Channel; 2018 [updated 2018 Apr 19; cited 2021 Mar 12]. Available from: https://www.betterhealth.vic.gov.au/health/healthyliving/immunisation-side-effects?viewAsPdf=true. [118] Victoria State Government, Better Health Channel. Fatigue [Internet]. Melbourne VIC: Victoria State Government, Better Health Channel; 2015 [updated 2015 Jun; cited 2020 Feb 12]. Available from: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/fatigue. [119] Victoria State Government, Better Health Channel. Fatigue [Internet]. Melbourne VIC: Victoria State Government, Better Health Channel; 2015 [updated 2015 Jun; cited 2020 Feb 12]. Available from: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/fatigue. [120] Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018 Apr 16;9:648. doi:10.3389/fimmu.2018.00648. [121] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [122] Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018 Dec;85(12):1118-1127. doi: 10.1007/s12098-017-2555-2. [123] Ring J, Klimek L, Worm M. Adrenaline in the acute treatment of anaphylaxis. Dtsch Arztebl Int. 2018 Aug 6;115(31-32):528-534. doi: 10.3238/arztebl.2018.0528.
0 Comments
Leave a Reply. |
AuthorSNotes from the team: Maxine, Monique and Bobby-Jo Archives
May 2023
|
 RSS Feed
RSS Feed
